

About nitrox fabrication (August 2022)
The development of the substitute energies to hydrocarbons
described in the previous food for thought topics has resulted in
many dives being performed at shallow depths where “nitrox” can
replace air to increase productivity and safety. For example, using
the COMEX table MT 92 and a mix of 40% oxygen, the equivalent
depth allows a no-decompression dive of 165 minutes at 18 m
instead of 50 minutes with air.
However, numerous companies do not apply these procedures
despite their advantage. One of the reasons is linked to the fact that
they are more complex to implement than air diving, and another
one is the price of oxygen.
The complexity of the implementation of nitrox is often linked to
personnel formation and is not discussed in this article. However,
difficulties related to the price of the oxygen can be partially solved
by fabricating nitrox mixes using specific membrane systems or
fabricating oxygen that can be used to compose nitrox by using
oxygen generators. Manufacturers say these devices can save up to
50% of the price of oxygen classically manufactured by cryogenic
separation.
Let’s start describing these machines with the fabrication of nitrox
using membrane systems:
1 - Description
Membrane systems selectively separate nitrogen and oxygen, from
the air. They have the advantages of fabricating nitrox at a low cost,
reducing logistical problems, and being easily transportable. They
can be classified into two main categories, which are not based on
the same principle of work.
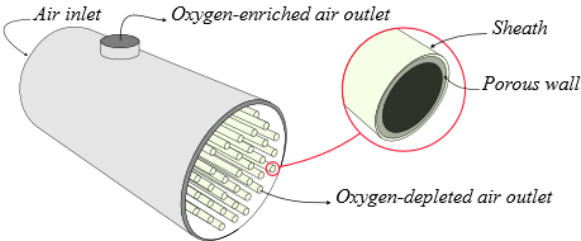
“Hollow fiber membranes” consist of hollow tubes made of fiber
manufactured by a co-extrusion-like process. The permeance of
gasses across the polymeric membrane is based on the solubility of
the gas in the polymer and the rate of gas diffusion across the
membrane. For these reasons, polymers are selected for the
membranes that are conducive to high permeance efficiency, light-
weight, and reliability.
The cross-section of a typical fiber has an outside diameter of 140-
180 microns and an inside diameter of approximately 100 to 140
microns. The majority of the fiber wall thickness is a porous
sponge-like material that makes up the fiber core. The purpose of
the core is merely to support an outer boundary layer of a thickness
of approximately 2 microns, called the sheath, where gas separation
occurs. The sheath and the outer skin of this layer, measured in
angstroms, determine the performance of the membrane.
These fibers are assembled in bundles to form the air separation
modules. The air is supplied at one end of each fiber and moves to
the other end. During this process, oxygen is absorbed through the
polymer walls of the fiber due to the pressure difference. As a
result, the gas that exits the downstream end of the hollow fiber is
decreased in oxygen concentration. Therefore, oxygen-enriched air
with oxygen concentration up to 40% is produced .
The advantages of this technology include the absence of moving
parts, the low weight, the inexpensive nature of the materials of
construction, and the lack of any substantial time lag in system
start-up.
The air inlet pressure is usually between 13 & 14 bar. This inlet air
should be filtered and dried to limit the particles size to 0.01µm, and
having a maximum oil vapour content below 0.01 mg/m³.
Several companies produce modules that are designed to be
compiled with adequate filtration systems and compressors. It is,
for example, the case of Parker, L’Air Liquide, Gereron, and others.
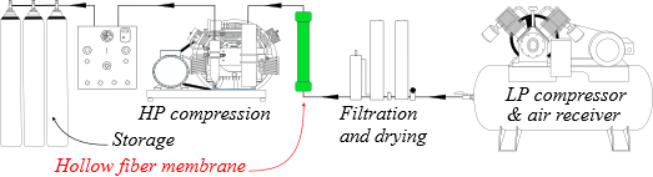
The scheme below shows how a nitrox production unit using these
modules should be organized.
Note that the NASA study “Onboard oxygen generation system” says
that that multi staging can improve the concentration of the oxygen
provided by such membrane systems.
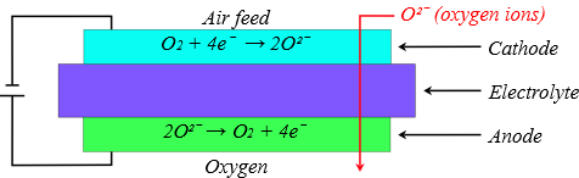
The second category of membrane separation systems is called
“ceramic membranes”. The principle of the ceramic oxygen
permeation process uses the catalytic properties of specialized
ceramic materials to transfer the oxygen in the form of ions instead
of molecules, so the ions of other gas molecules cannot pass
through the membrane. As a result, the oxygen concentration can
reach 99.5% or even higher. The system is based on a membrane
where one side is the cathode, and the other is the anode that is
separated from the cathode by an electrolyte. Oxygen molecules'
absorption starts at the membrane's cathode side, where they are
dissociated into oxygen ions. These oxygen ions migrate to the anode
side through the membrane and then recombine into oxygen
molecules. The compensation of electric charges during the process
is achieved through reverse-direction migration of the electrons in
an external electric circuit. Note that the operating temperature of
ceramic membranes is between 600 and 900 °C.
Like hollow fiber membranes, ceramic membrane systems do not
use moving parts. In addition, they have the advantage of not being
sensitive to water vapour and other contaminants. Nevertheless,
these devices need to be energized by an electric circuit to work.
These systems are fabricated mostly for aerospace and defense
industries.
2 - Design and operating procedures of a system
Like for other types of equipment, it is essential to study an existing
system's design and operating procedures to understand better
how such equipment can be adapted to commercial diving activities.
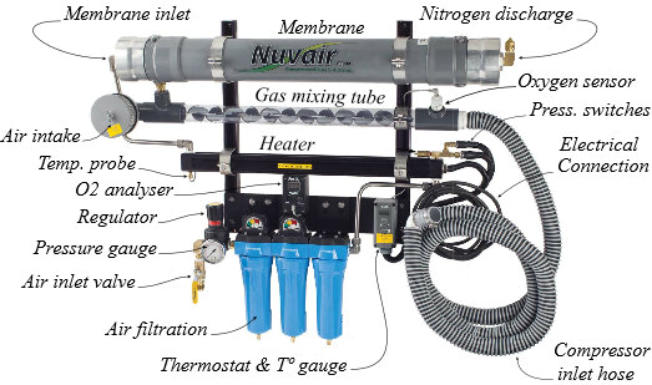
The device taken as a reference is the "230n3 Series" created by
"Nuvair" (https://www.nuvair.com/), a company based in Oxnard,
California, USA. This system, which is among the most sold, can
supply nitrox with an oxygen concentration of up to 40%.
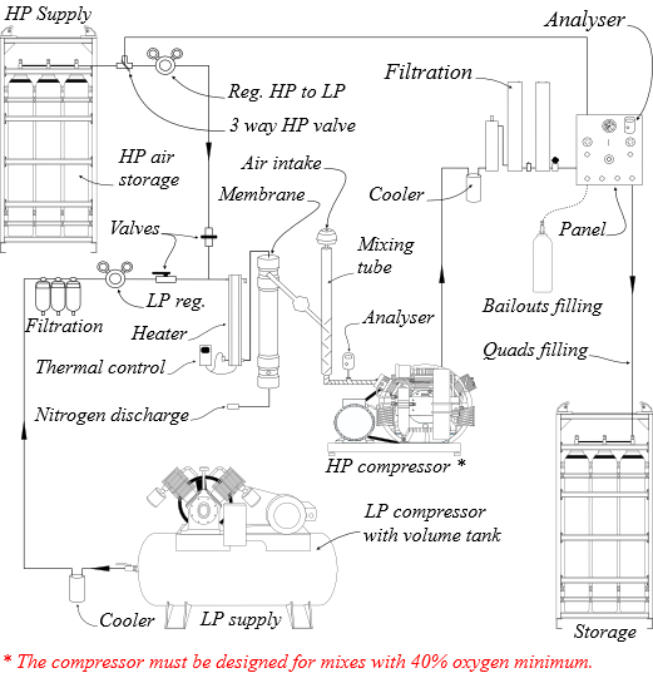
1.
The Membrane Systems require a source of clean,
pressurized, and heated air for separation. The two most
common sources are a Low Pressure Compressor (LP Supply)
or High Pressure air storage tanks (HP Supply).
2.
The air must be properly filtered to be “oxygen compatible”
quality prior to entering the membrane system so it will not
damage or plug the membrane fibbers. Standard systems are
rated for maximum supply pressures of 17 bar (250 psi) for
LP Supply and 345 bar (5000 psi) or sometimes more for HP
Supply.
3.
An “input pressure regulator” reduces these pressures to
acceptable levels for the membrane.
4.
The air is then heated to a temperature that provides stability
over a wide range of ambient conditions and is optimal for
membrane permeation.
5.
The heated air enters the membrane, which is made up of
thousands of miniature hollow fibers. The walls of these fibers
are semi-permeable and designed for different gases to move
through them (or permeate) at different speeds. The resulting
gas mixture is known as the “permeate”.
6.
As air flows through the hollow fibers, both oxygen and
nitrogen permeate through the fiber walls. The oxygen
permeates faster than the nitrogen, which produces permeate
with an oxygen content greater than air. The gas that reaches
the end of the hollow fibers without permeating is almost
entirely nitrogen and is discharged. The flow rate of this
discharge is set by the factory via a fixed orifice to allow the
membrane to operate at maximum volume and efficiency. The
resulting permeate contains approximately 40% O2 and is
constant under all operating conditions.
7.
The permeate is a concentrated mixture that must be diluted
with additional air prior to entering the nitrox compressor. It
exits the membrane at ambient to slightly negative pressure
and travels into the “mixing tube”, where it mixes
homogeneously with filtered outside air. The amount of dilution,
and thus final % O2 , is obtained by adjusting the “input
pressure regulator”: As pressure is increased, permeate flow
increases, air flow decreases, and a higher % O2 Nitrox is
produced. As pressure is decreased, permeate flow
decreases, air flow increases, and a lower % O2 Nitrox is
produced. This relationship between permeate flow and air
flow exists because the total of these two flow rates will always
equal the intake flow rate demanded by the Nitrox Compressor.
8.
The resulting nitrox mixture is analysed for approximate % O2
before entering the nitrox compressor and again prior to use
for precise % O2 .
9.
The input pressure that correlates to a specific nitrox % O2 is
repeatable. If nitrox with 36% O2 is produced when the input
pressure is at 9 bar (125 psi), then adjusting the Regulator to
the same pressure during the next use will produce the same
gas mixture.
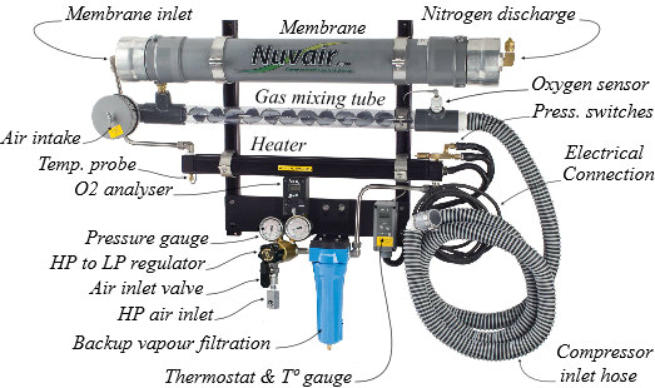
Identification of the components of a system supplied with low
pressure:
Identification of the components of a system supplied with high
pressure:
Overall view of the components of a system supplied with high and
low pressure:
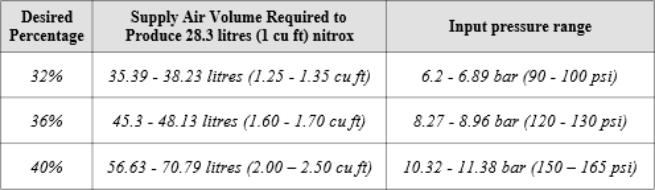
As said previously, the higher the % of O2 desired in the final
product, the greater the volume of supply air, and the higher the
input pressure required, as shown in the example below for a 10
cfm (283 L/min) membrane system:
The air supplied should comply with EN 12021 or CGA G-7.1-1997
grade D or E.
Manufacturers of nitrox membrane systems also sell complete
ensembles that include the compressor, such as the machine below
designed by Nuvair that can produce up to 481 litres/min of nitrox
40 % (max. pressure: 250 bar).
3 - Where to install the machine?
Like all air compressors used on worksites, the machine's air intake
should be placed at height and away from any potential sources of
dangerous gas. In addition, the area where it is installed should be
cleared of pollutants that may ignite oxygen such as oil and grease.
Thus, a risk assessment regarding its surrounding should be done.
Also, nitrox mixtures used for diving usually have proportions of
oxygen above 25%, so they must be handled as pure oxygen. The
document IMCA D 022 chapter 9/point 9.6 recommends not to
pump oxygen. For this reason, many companies transfer pure
oxygen and nitrox mixes by decanting only during operations at sea,
and some others buy mixes fabricated in factories.
However, a lot of companies have ceased to apply this guideline and
argue that, if relevant precautions are implemented, it is no riskier to
pump nitrox mixes offshore than implementing other operations that
are commonly done, such as fuel bunkering or the gas containers
transfer by crane. We must admit that accidents with these
machines are rare, as we have not found a recent paper regarding
such events.
For this reason, we can consider that pumping nitrox mixes is
possible if a risk assessment has been undertaken to ensure the
desirability of doing it, that the fire surveillance and fire fighting
systems are sufficient, and that this operation is performed in an
isolated ventilated part of the deck where a fire can be easily and
quickly contained. In addition to extinguishers and fire lances in
immediate proximity to the room, the fire fighting systems should
include a deluge or a water mist system with a fire alarm system
linked to the vessel's bridge,
Of course, the vessel owner, the client, and the state representative
can reject such a procedure.
Thanks to Erick Estrada & Ron Case from NUVAIRr for their
kindness and efficient support.


Purpose and potential applications:
Membrane gas separation systems used to extract the oxygen, such
as the nitrox membrane system Nuvair described in the previous
post (see in discussion “August 2022”) or models from other
manufacturers, provide nitrox mixes limited to approximately 40%
oxygen. However, it is possible to obtain nearly pure oxygen with
technologies such as Pressure Swing Absorption (PSA) oxygen
generators. In recent decades, this technology has improved to
become efficient, reliable, transportable, and financially accessible.
Like the nitrox membrane system described in the previous section,
these apparatus extract oxygen from the natural air. The purity
obtained ranks from 90 to 99.5%, depending on the equipment. Note
that 90% is the minimum required by standardization organizations.
Pressure Swing Adsorption oxygen generators are primarily used
for medical support, particularly for mobile hospitals and those
installed in isolated areas. They are also increasingly installed to
reduce the cost of therapeutic gasses in hospitals established in
towns and provide oxygen treatment for individuals at home.
However, the study of their working process proves that they can
be employed for other applications, such as the production of nitrox
mixes.
For remembering, 99.5% is the minimum recommended oxygen
purity of the European standard EN 12021, US Navy, and others. For
this reason, oxygen not complying with this minimum must not be
used for decompression and therapeutic treatments, as the tables
have been calculated according to this minimum oxygen purity. Thus,
except if the oxygen produced conforms with the above, these
apparatus cannot be used to supply pure oxygen.
However, nothing prevents us from using oxygen extracted from the
air with less than 99.5% purity for nitrox mixes, considering that the
remaining 10 to 1% of impurities of the oxygen produced by these
apparatus are nitrogen and argon.
As a result, oxygen with more than 99.5% purity can be kept for
decompression and medical treatment, and nitrox can be produced
with oxygen extracted from the air without affecting these reserves.
Note that most machines currently available on the market produce
oxygen with a purity between 93 & 97%. However, a few
manufacturers are able to sell machines producing oxygen with a
minimum purity of 99.5%.
Description:
Molecular sieves used with “Pressure Swing Adsorption (PSA)”
systems are crystalline synthetic or naturally occurring zeolites
(aluminosilicate minerals with microporous structure) with pores of
precise and uniform size that have the capacity to absorb and
separate gasses and liquids. The absorption and separation of the
molecules are based on the size of the molecules, so only small
enough ones can enter the pores, and it is also based on their
electric charge (electro-static fields). Molecular sieves are classified
according to their chemical formula and pore sizes. They are used
for applications such as drying gases, absorbing undesirable
gasses such as ammonia, methanol, ethanol, carbon dioxide,
hydrogen sulfide, and fabricating gasses such as oxygen, nitrogen,
or hydrogen.
Molecular sieves type 13X are commonly used to separate nitrogen
from the air to produce oxygen. They are the sodium form of the
aluminosilicate molecular sieves with pore diameters of
approximately 10 angstroms, an external diameter between 0.4 to
2.5 mm, and a light grey colour. They can be thermally regenerated
at temperatures from 180 °C to 300 °C. Another regeneration
method consists of gradually reducing the applied pressure.
Zeolites of Pressure Swing Adsorption systems have the
inconvenience of increasing the proportion of argon in the oxygen
delivered. As an example, for a system delivering a mix with 93%
oxygen purity, the ratio of argon is 4% instead of 1% in the
atmosphere. For this reason, NASA studies on onboard oxygen
generation systems recommend using a 2nd bed made of carbon
to absorb the excess argon.
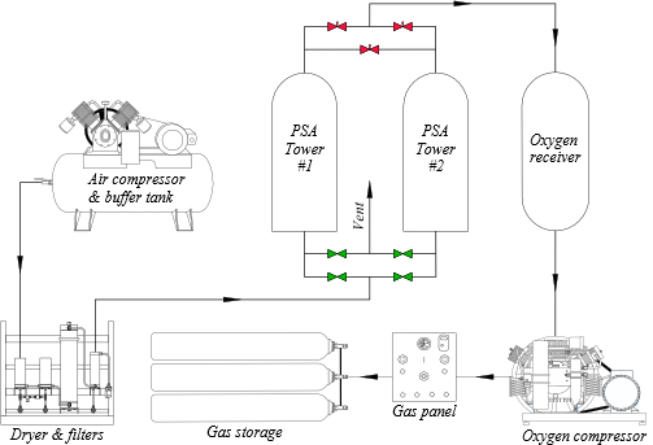
Most systems use two zeolites towers so that one unit is in use
when the molecular sieves in the second unit are regenerated. The
basic scheme of the systems commonly used is similar to the one
displayed below.

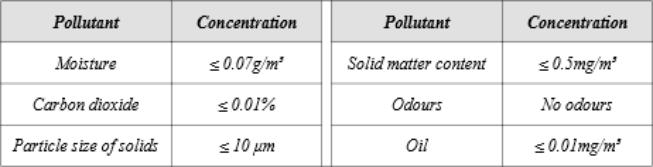
Note that the air from the compressor should be oxygen compatible.
The following specifications are commonly asked:
Oil free screw air compressors are known to deliver high flow of
low pressure air, and are often preferred to supply the installations.
Also, most installations are provided with a low pressure storage
tank.
As an example of machine that can be adapted for the production of
nitrox, the Pressure Swing Adsorption (PSA) Oxygen generator
model NZO-30 PSA below, designed by Hanghou Nuzhuo Technology
co, Ltd (https://www.hznuzhuo.com/), can deliver 30 m3/hr of
oxygen with 95% purity, so 3 cylinders 50 litres/200 bar/hour. It is
made of the following components:
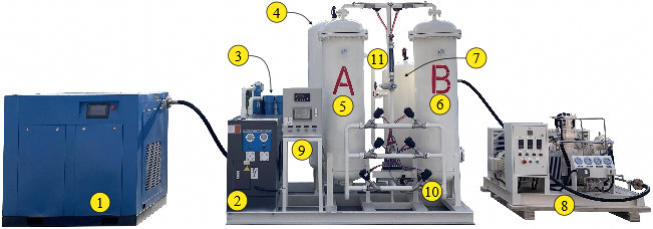

1 - Screw compressor 7.5 m3/min
2 - Air purification: Dryer 6
m3/min
3- Air purification: Filtration
4 - Air buffer tank
5 - Absorption tower “A”
6 - Absorption tower “B”
7 - Oxygen buffer tank
8 - Oxygen compressor 30 m3/h
9 - Controller
10 - Valves
11 - Flow meter
12 - Manifold for filling
This equipment was initially designed to be installed in ventilated
rooms with a surface of at least 30 m² and a height not less than
4 m.
However, Hanghou Nuzhuo Technology provides the photo above that
shows that this machine can be installed in a 20 feet container,
provided that adequate ventilation and fire fighting systems are in
place.
The manufacturer recommends to keep the machine at an ambient
temperature of not less than 5 ºC and not more than 49 ºC. Low
noise fans directing the hot air to the outside of the room are
suggested for this purpose and to avoid the accumulation of oxygen
in the room. Note that the heat and gas accumulation problems that
may arise due to lack of space in offshore containers can be
efficiently compensated by providing large top and bottom openings,
ensuring adequate air circulation. Usually, such openings are
provided with louvers so they can be kept open when it rains.
Waterproof external shutters are typically provided to close these
openings when the machine is not used and transferred to another
place.
Note that the controller allows managing the oxygen production and
sieve regeneration automatically.
This machine is the less powerful of the range sold by the
manufacturer taken in reference. It allows providing the necessary
oxygen (95% purity) for 24 hours diving operation at 18 m using a
mix 50/50 and 1 diver in the water within less than two hours of
compression. However, many diving operations do not need so
powerful machines, and the space available on the surface support
may not be sufficient to accommodate a container like the one
above.
Some manufacturers specialize in less powerful and more
transportable machines initially designed for small hospitals and
individuals. It is the case of the models developed by OXUS
(https://www.oxus.co.kr/en/), a company based in Korea that sells
a range of machines installed in cabinets mounted on castors.
These cabinets accommodate several small appliances that can be
switched on and off, depending on the volume of oxygen to
fabricate. For example, the model RAK-U06M2E below is composed
of an ensemble of six small oxygen generators, each of which can
work independently and two control units.
The size of the cabinet (W x D x H) taken in reference is 960 x 600
x 1,550 mm. Optional oxygen storage tanks are provided on the top
of the cabinet. However, the system is designed to supply hospital
circuits at the outlet pressure delivered (4 - 5 bar), and an HP
compressor is to be added to the machine to fill HP gas cylinders.
OXUS provides a high pressure booster that can compress the
cylinders to a maximum pressure of 150 bar for this purpose.
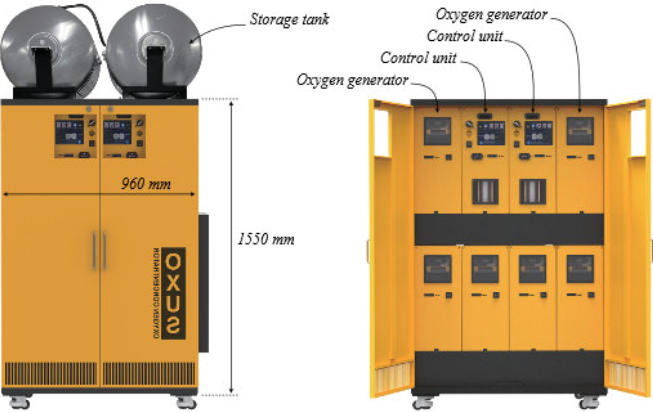
This system can fabricate 3600 l of oxygen per hour, so 89400 l
per day.
Note that smaller units are also available on the market. For
example, the manufacturer of the model described above sells a
machine designed to provide 43200 litres per 24 hours and
another tiny unit designed for 7200 litres per day. Even though this
last machine is unsuitable for the fabrication of oxygen for the
diving team, it can be used in the onboard hospital for oxygen
supports other than hyperbaric treatments.
Implement the machine:
Using such machines on worksites is a new idea. However,
considering that they are successfully used for hospitals in isolated
areas, there is no reason for not implementing them, provided that
the precautions indicated by the manufacturers and above are
implemented. These precautions include the position of the air inlet
of the compressor and the elements already discussed for the
implementation of membrane systems for nitrox fabrication.
Thus, a risk assessment should be done to ensure that the
machine's surroundings are safe, and that relevant firefighting
systems are ready for use.
Of course, the vessel owner, the client, and the state representative
can reject using such a machine onboard the ship.
In addition, using the oxygen produced by such a machine to
fabricate various nitrox implies that the personnel implementing it
and then performing mixing of the oxygen with air to make the
desired nitrox must have a relevant formation.

Click on the
octopus to return to
the top of the page











